Radio Carbon Dating Formula
Radioactive decay is a statistically random process, just like a roulette wheel in a casino is. For a given isotope there is a fixed probability per unit of time that an atom will decay.
Of course, the atom may decay in a second, a day, a year or a millenium. But what you have to consider is this probability averge over all of the atoms in a sample. In a mole of atoms there are some 6 x 10^23 atoms. So if I now calculate the average time for 1/2 of those atoms to decay based on the average rate of decay above and its standard deviation, I will get a very, very precise number with almost no variation at all. This number is called the half life, and is what is measured.
For carbon 14 it is about 5568 years (the error in knowing it is that it is hard to take measurements for 5568 years, so we have to estimate from shorter observations!). The rate at which carbon 14 is produced is not constant, and this is an issue for carbon dating. This is overcome by calibrating against items with a known date.
So trees whose rings can be counted are really useful, as are objects found with archaeological artifacts whose dates are known. Radio-carbon dating is a method of obtaining age estimates on organic materials. The word 'estimates' is used because there is a significant amount of uncertainty in these measurements.
Sep 11, 2009 - 13 minCarbon dating is a real-life example of a first-order reaction. This video explains half-life in the. Dec 3, 2010 - 10 minCarbon 14 Dating 1. The ratio of carbon 14 to carbon 12 can indeed be changed due to.
Each sample type has specific problems associated with its use for dating purposes, including contamination and special environmental effects. More information on the sources of error in carbon dating are presented at the bottom of this page.
The method was developed immediately following World War II by Willard F. Libby and coworkers and has provided age determinations in archeology, geology, geophysics, and other branches of science. Radiocarbon dating estimates can be obtained on wood, charcoal, marine and freshwater shells, bone and antler, and peat and organic-bearing sediments. They can also be obtained from carbonate deposits such as tufa, calcite, marl, dissolved carbon dioxide, and carbonates in ocean, lake and groundwater sources. Carbon dioxide is distributed on a worldwide basis into various atmospheric, biospheric, and hydrospheric reservoirs on a time scale much shorter than its half-life. Measurements have shown that in recent history, radiocarbon levels have remained relatively constant in most of the biosphere due to the metabolic processes in living organisms and the relatively rapid turnover of carbonates in surface ocean waters. However, changes in the atmosphere over the ages are a source of uncertainty in the measurements.
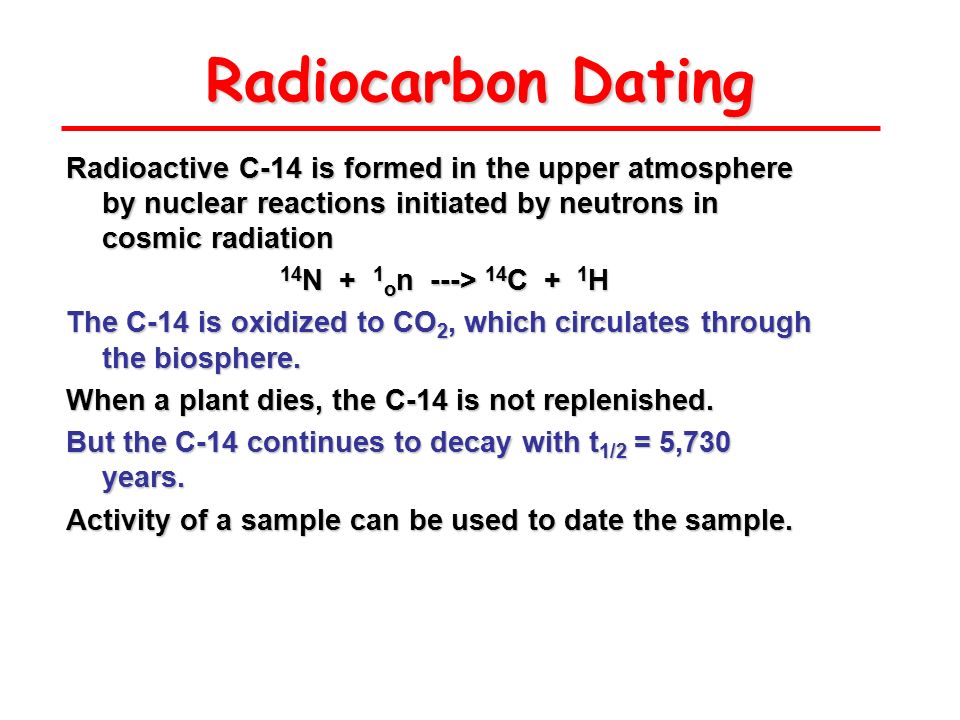
Carbon (C) has three naturally occurring isotopes. Both C-12 and C-13 are stable, but C-14 decays by very weak beta decay to nitrogen-14 with a half-life of approximately 5,730 years.
Naturally occurring radiocarbon is produced as a secondary effect of cosmic-ray bombardment of the upper atmosphere. Plants transpire to take in atmospheric carbon, which is the beginning of absorption of carbon into the food chain.
Animals eat the plants and this action introduces carbon into their bodies. After the organism dies, carbon-14 continues to decay without being replaced. To measure the amount of radiocarbon left in a artifact, scientists burn a small piece to convert it into carbon dioxide gas. Radiation counters are used to detect the electrons given off by decaying C-14 as it turns into nitrogen.
The amount of C-14 is compared to the amount of C-12, the stable form of carbon, to determine how much radiocarbon has decayed, thereby dating the artifact. Exponential Decay Formula: A = A0* 2^(-t/k) Where 'A' is the present amount of the radioactive isotope, 'A0' is the original amount of the radioactive isotope that is measured in the same units as 'A.' The value 't' is the time it takes to reduce the original amount of the isotope to the present amount, and 'k' is the half-life of the isotope, measured in the same units as 't.' Uncertainty in Carbon Dating As mentioned above, there is significant uncertainty in carbon dating. There are several variables that contribute to this uncertainty. First, as mentioned previously, the proportions of C-14 in the atmosphere in historic times is unknown.
The C-14:C-12 atmospheric ratio is known to vary over time and it is not at all certain that the curve is “well behaved.” Complicating things further, various plants have differing abilities to exclude significant proportions of the C-14 in their intake. This varies with environmental conditions as well. The varying rates at which C-14 is excluded in plants also means that the apparent age of a living animal may be affected by an animals diet. An animal that ingested plants with relatively low C-14 proportions would be dated older than their true age. Attempts are often made to index C-14 proportions using samples of know age.
While this may be useful to eliminate the uncertainty of atmospheric proportions of C-14, it does not compensate for local conditions such as which plant species are in the diet. The uncertainty in the measurement leads some to conclude that the method is far less predictive of age than is commonly supposed, especially for older samples. • Tell us some more • Upload in Progress • Upload failed. Please upload a file larger than 100x100 pixels • We are experiencing some problems, please try again. • You can only upload files of type PNG, JPG, or JPEG. • You can only upload files of type 3GP, 3GPP, MP4, MOV, AVI, MPG, MPEG, or RM.
• You can only upload photos smaller than 5 MB. • You can only upload videos smaller than 600MB.
• You can only upload a photo (png, jpg, jpeg) or a video (3gp, 3gpp, mp4, mov, avi, mpg, mpeg, rm). • You can only upload a photo or a video. • Video should be smaller than 600mb/5 minutes • Photo should be smaller than 5mb •.
Atmospheric carbon dioxide, CO 2, has a steady-state concentration of about one atom of carbon-14 per every 10 12 atoms of carbon-12. Living plants and animals that eat plants (like people) take in carbon dioxide and have the same 14C/ 12C ratio as the atmosphere.
However, when a plant or animal dies, it stops taking in carbon as food or air. The of the carbon that is already present starts to change the ratio of 14C/ 12C. By measuring how much the ratio is lowered, it is possible to make an estimate of how much time has passed since the plant or animal lived.
The decay of carbon-14 is: 14 6C → 14 7N + 0 -1e (half-life is 5720 years) Example Problem A scrap of paper taken from the Dead Sea Scrolls was found to have a 14C/ 12C ratio of 0.795 times that found in plants living today. Estimate the age of the scroll. Solution The of carbon-14 is known to be 5720 years. Radioactive decay is a first order rate process, which means the reaction proceeds according to the following equation. Helmenstine, Anne Marie, Ph. Meksikanske Voksne Bare All Inclusive Resorts. D.
'Carbon 14 Dating of Organic Material. Tipps Und Tricks Ebay. ' ThoughtCo, Feb. 8, 2017, thoughtco.com/carbon-14-dating-of-organic-material-609545. Helmenstine, Anne Marie, Ph.D. (2017, February 8). Carbon 14 Dating of Organic Material. Retrieved from Helmenstine, Anne Marie, Ph.D.
'Carbon 14 Dating of Organic Material.' (accessed December 4, 2017).